Adhesive Bonding & Joining
Adhesive Failure, Plastics Adhesion Bonding
Adhesive failures and adhesion failures occur at the interface of two similar or dissimilar substrate surfaces. Bonding processes involve both adhesive and cohesive forces. The type of adhesion failure can be characterized by adhesive, cohesive, or substrate failure, depending on the strength of these forces. Adhesive failure, or delamination, generally is caused by the characteristics of the substrate, including its surface chemistry and surface energy, surface preparation and application procedures. Cohesive failure relates to the internal strength of the adhesive. Substrate failure is not related to the bonding process, rather the strength of the substrate itself. The bond is stronger than the actual substrate.
Adhesion problems can be rapidly solved with process engineering experts skilled in the fields of polymers and metals, chemistry, adhesives, primers, coatings, silanes, plasma pretreatments, surface quality, molding operations, and testing methods.
The Sabreen Group achieves outstanding success solving complex 2-dimensional and 3-dimensional bonding problems using our advanced technologies and Six Sigma process methods. We develop innovative solutions for a broad spectrum of applications, including jet aircraft fighters, medical, automotive, electronics, consumer products, and more. One of our patented processes was employed to manufacture “smart bomb” weapon systems during Operation Desert Storm and continues to be used as a standard of bonding excellence for military and NASA applications. Another technology enables exceptional waterborne coating adhesion performance on polyolefin products for exterior housing applications.
SABEEN’s technologies are employed to manufacture many of today’s most recognizable military and commercial products, from the International Space Station and pharmaceuticals to underwater action cameras. SABREEN’S engineering has earned top industry awards for our clients, including pharmaceutical/medical packaging product of the year, inducted into the Smithsonian National Museum. Our early project involvement achieves accelerated project schedules.
SABREEN's innovative solutions for applications include:
- Adhesives & Sealants
- Plastic to Plastic
- Elastomers & Rubbers
- Inkjet & Printing Inks
- Coatings & Paints
- Metallization & Plating
Custom bonding & curing technologies for hermetic sealing of polyolefins
Custom pretreatment, inkjet chemistry & UVLED Cure
Plasma pretreatment & custom ink on PPS lock safe dial
Breakthrough inkjet technology for elastomeric wearables
Optomechanical bonding anti-fog/AR/hardcoat on Polycarbonate
Plasma pretreatment for polyolefin closures
Flame lamination for automotive seating
Award winning pharma/medical fashionable birth control carrying cases
Polymer roofing tiles decorated/coated to simulate slate, cedar shake, 40-year warranty lightfastness, fire & wind
Full color digital printing with FDA chemical resistant clear topcoat for dishwasher stability
Custom decoration & special-effect top coatings simulate soft-touch & woodgrain
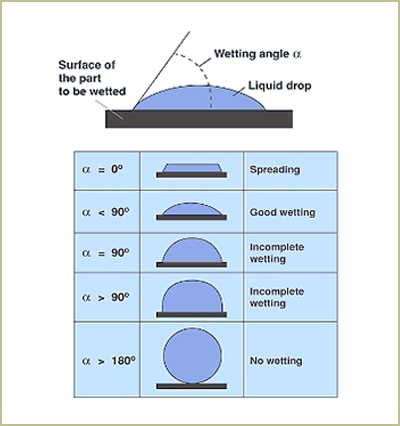
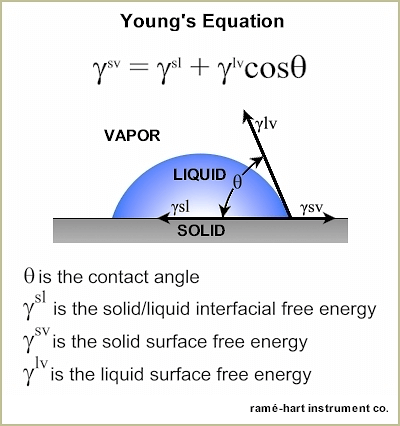
The contact angle is the angle typically measured through the liquid, where a liquid-vapor interface meets a solid interface. The contact angle quantifies the wettability of a solid surface by a liquid via the Young equation.
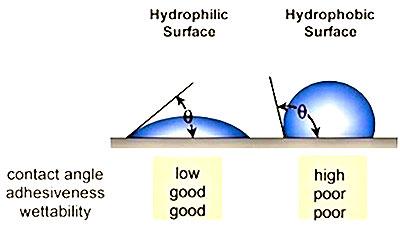
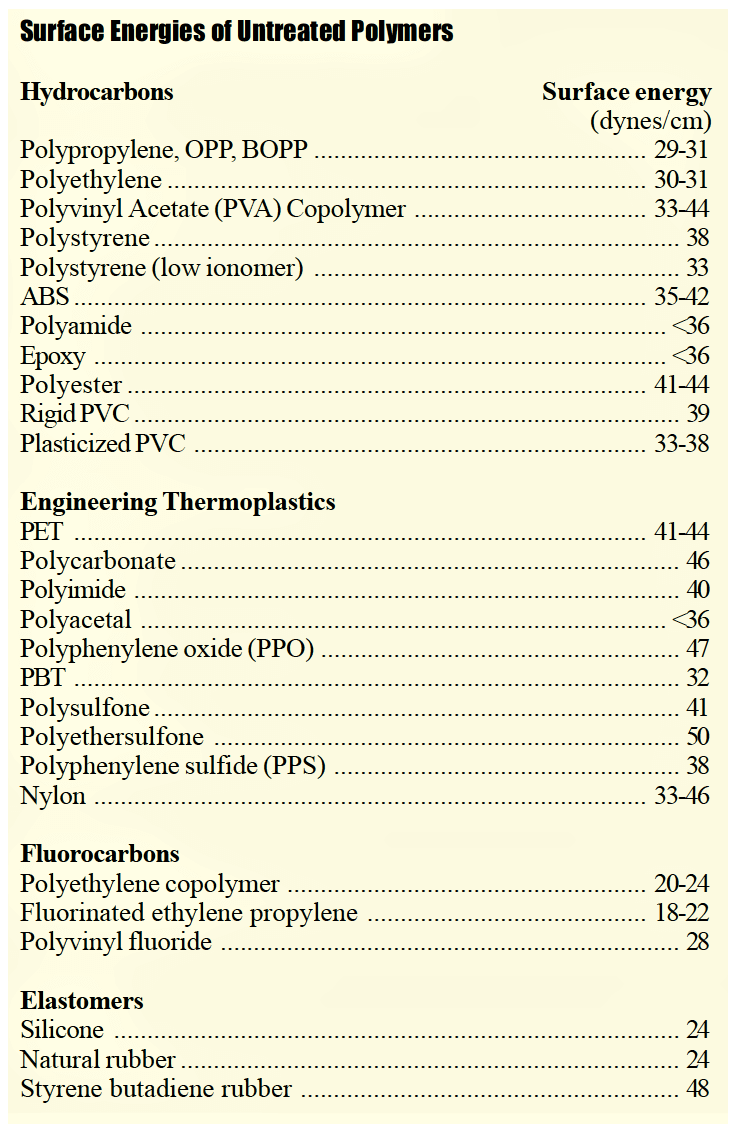
The underlying reasons why many plastics are difficult to bond are because they are hydrophobic non-polar materials, chemically inert and possess poor surface wettability – i.e., low surface energy. While these hydrophobic (repel water) performance properties are ideal for part designers who seek such properties, they are the nemesis for manufacturers needing to bond such materials. Robust adhesion bonding usually necessitates “hydrophilic” surfaces. For optimum adhesion to occur, an adhesive (coating, ink or paint) must thoroughly “wet out” the surface (adherend) to be bonded.
“Wetting out” means the liquid flows and covers a surface to maximize the contact area and the attractive forces between the adhesive and adherend bonding surface. For a liquid to effectively wet out a surface, the surface energy of the adhesive must be as low or lower than the surface energy of the adherend to be bonded. Alternatively, the surface energy of the substrate must be raised.
Consider a single liquid fluid droplet on a flat solid surface at rest (equilibrium). The angle formed by the solid surface and the tangent line to the upper surface at the end-point is called the contact angle; it is the angle (?) through the liquid between the tangent line at the contact point and the horizontal line of the solid surface. The bubble/droplet shape is due to the molecular forces by which all liquids, through contraction of the surface, tend to form the contained volume into a shape having the least surface area. The intermolecular forces that contract the surface are termed “surface tension.” Surface tension, a measurement of surface energy, is expressed in dynes/cm (SI N/m).
The higher the surface energy of the solid substrate relative to the surface tension of a liquid (water, printing inks, adhesives/encapsulation, coatings, etc.), the better will be its “wettability” and the smaller will be the contact angle (Figure 1 on page 2). As a rule, acceptable bonding adhesion is achieved when the surface energy of a substrate is approximately 8 to 10 dynes/cm greater than the surface tension of the liquid.
There is a strong tendency for manufacturers to focus only on contact angles or other wettability measurements as the sole predictor for bonding problems and for conducting routine surface testing. Chemical surface functionality is equally important, whereby hydrophobic surfaces are activated into bondable hydrophilic surfaces. Gas-phase, “glow-discharge,” surface oxidation pretreatment processes are used for chemical surface activation. These processes are characterized by their ability to generate “gas plasma,” an extremely reactive gas consisting of free electrons, positive ions and other species. Plasmas often can be described as a fourth state of matter. As energy is supplied, solids melt into liquids, liquids vaporize into gases, and gases ionize into “plasmas.”
In the science of physics, the mechanisms in which these plasmas are generated are different, but their effects on surface wettability are similar. The basic chemical and physical reaction that occurs is free electrons, ions, metastables, radicals and UV generated in the plasma can impact a surface with energies sufficient to break the molecular bonds on the surface of most polymeric substrates. This creates very reactive free radicals on the polymer surface that, in turn, can form, cross-link, or in the presence of oxygen, react rapidly to form various chemical functional groups on the substrate surface. Polar functional groups that can form and enhance bondability include carbonyl (C=O), carboxyl (HOOC), hydroperoxide (HOO-), and hydroxyl (HO-) groups. Even small amounts of reactive functional groups incorporated into polymers can be highly beneficial to improving surface chemical functionality and wettability. Also, chemical primers/solvents and mechanical abrasion (including mold tool texture) can be utilized alone or in conjunction with pretreatments.
The degree or quality of pretreatment for robust adhesion strength is affected by the cleanliness of the plastic surfaces. The surface must be clean to achieve optimal pretreatment and subsequent adhesion. Contamination sources on product surfaces that inhibit treatment includes dirt, dust, grease and oil. Low molecular weight materials such as silicones, mold release and anti-slip agents are particularly deleterious for bonding. Material purity is also an important factor. Further, certain soluble or nonsoluble compound agents used in pigment and dye colorants can adversely affect adhesion.
The shelf life (treatment aging degradation) of treated plastics depends on the type of resin, formulation and the ambient environment of the storage area. Shelf life of treated products is limited by the presence of low molecular weight oxidized materials (LMWOM) such as antioxidants, plasticizers, slip and antistatic agents, colorants and pigments, stabilizers, etc. Exposure of treated surfaces to elevated temperatures increases molecular chain mobility. The higher the chain mobility is, the faster the aging of the pretreatment. Plasma-treated surfaces age at different rates and to varying extents relative to factors with the surrounding environment. Aging characteristics and their storage shelf life are essential to manufacturing process operations. Activated surfaces may have a shelf life of hours, days, months or longer. It is recommended to bond, coat, paint or decorate products as soon as possible following pretreatment.
Recognize that each surface pretreatment method is application-specific and may possess unique advantages and potential limitations. For robust results, consider the following factors:
- Polymers react differently to oxidation processes. The type of polymeric substrate and its end-use performance requirements are critical in determining the selection of pretreatment method.
- Is the substrate (product to be treated) conductive? For example, unassembled plastic electronic connector bodies – without metal contact pins – can be treated electrically, whereas assembled connectors may experience electrical arcing problems.
- Part geometry – flat surfaces are more easily treated compared to deep recesses, extreme tapers, and other shape irregularities. Wetting tests are difficult to conduct in small areas and on heavily textured surfaces.
- Material handling automation: Conductive belts and chains may cause electrical arcing with classical electrical corona discharge and spot treaters. Alternatively, consider using flame, cold gas plasma, or low-potential atmospheric plasma.
- Avoid overtreatment. Excessive plasma-oxidized surfaces may deleteriously affect downstream assembly processes such as heat sealing/welding.
- All pretreatment equipment is not created equal. Examine the quality of constructed systems in action. For electrical treatment processes, observe the uniformity of the plasma discharge; for flame treatment, consider the differences between ribbon vs. drilled port burners and combustion system components; for cold gas plasma, examine the quality of the chamber construction, electrode shelves and particularly the manufacturer of the vacuum pump.
Best method for inspecting surface quality – The Surface Analyst™ 2001 is a handheld digital device for measuring contact angles in-line production without using chemical dyne solutions. The method is nondestructive; thus the part can be processed as usual – unlike dyne solution testing in which the part is scraped. Invented by BTG Labs, the Surface Analyst™ was engineered to put precision surface measurement technology in the hands of shop-floor technicians for operations where surface condition is critically important, such as adhesive bonding, painting, coating, printing, and decontamination.
Using a patented technique called “ballistic deposition,” the device deposits a highly purified drop of water on the substrate surface. By knowing the volume and area of a drop of water, the device then calculates the contact angle of the water against a given surface. The device provides immediate feedback to the user with a quantifiable number or a Pass/Fail alert. The inspection data provided by the device allows users to determine if the substrate has been properly prepared for adhesion processes as a replacement for destructive dyne solutions. The handheld unit operates in any orientation and works on smooth or textured surfaces, such as grained TPOs. This data can be used for continuous process improvement, identifying quality drifts that may signal imminent problems, or to pinpoint the critical cause of product failures.

For many applications it may only be necessary to examine the static equilibrium contact angle using dyne solutions in accordance to a documented test procedure. Application kits or “dyne pens/solutions” provide useful information, but they are not precise measurements of surface tension. Surface tension measurements can vary considerably by individual (technique) and the interpretation of the “center” liquid behavior. Dyne pens/solutions are known to be directional indicators of significant differences in the surface tension and capable of identifying “good” and “bad” bondable surfaces at economical pricing. Bottled dyne solutions can be preferable instead of pens because of contamination issues due to multiple usage.
Optimal joint design is critical in any adhesive bonding application. Bonded joints can be subject to tensile, compressive, shear, peel or cleavage forces, often in combination. There are generally three distinctive types of bonding failure mechanisms:
- Adhesion failure interfacially between the adhesive and one of the adherends
- Cohesion failure by fracture of the adhesive layer leaving traces of adhesion on both surfaces
- Mixed-mode failure exhibits characteristics of both adhesion and cohesion failure
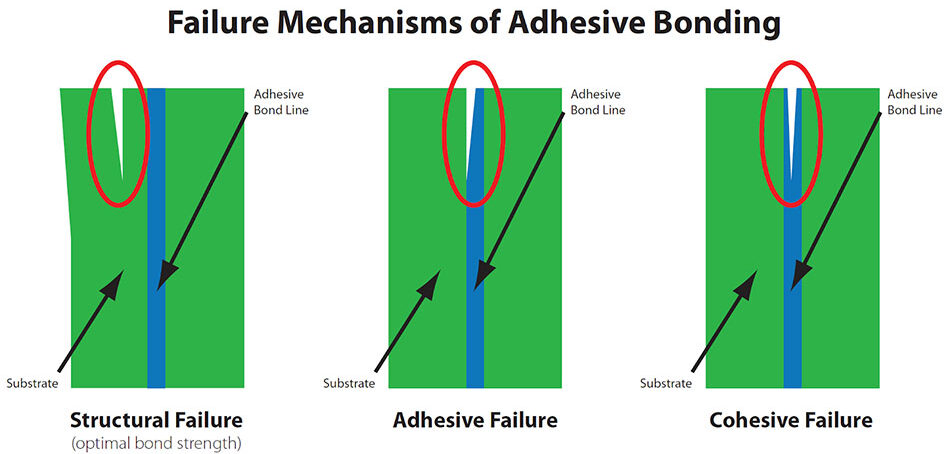
Adhesion failures occur at the interface between the adhesive and the adherend (substrate). Visually, there will be residual adhesive remaining at any location on only one surface. Adhesion failures that occur in-the-field almost always originate in the manufacturing process; materials, adhesives, surface preparation, contamination, procedures/workmanship, environment, quality control testing and combinations thereof. The remaining cause is interfacial degradation, as a function of field service life. This latter problem can be prevented during the product design/qualification phase.
Cohesion failures result in fracture of the adhesive and are characterized by the visual presence of the adhesive material on the matching faces of both adherends (substrates). Bonds which fail by cohesion exhibit high-strength performance (achieving optimal dry adhesive bond properties). The adhesive surface may appear “rough” and may be lighter in color than the adhesive material, as applied. Failure is usually by shear, but peel stresses or a combination of shear and peel can produce cohesion failure. Cohesion failures that occur in-the-field are typically caused by poor joint design, e.g., insufficient overlap length, or excessive peel forces. Excessive porosity (humidity exposure), and the presence of voids below a critical size can also result in cohesion failure.
Mixed-mode failures exhibit characteristics of both adhesion failure and cohesion failure because the interface is partially degraded. Essentially, this failure mode is a transitional phase in which the strength of the adhesive bond is lower than the cohesion failure strength.
Surface roughness is the degree of unevenness of the surface of a solid below the scale of magnitude of its shape or undulation but above the irregularity of crystal lattice structures. The degree of roughness affects the wettability of a solid. As roughness goes hand-in-hand with an enlarged surface area, it affects the wettability of a solid, the contact angle of a liquid, and the adhesion.
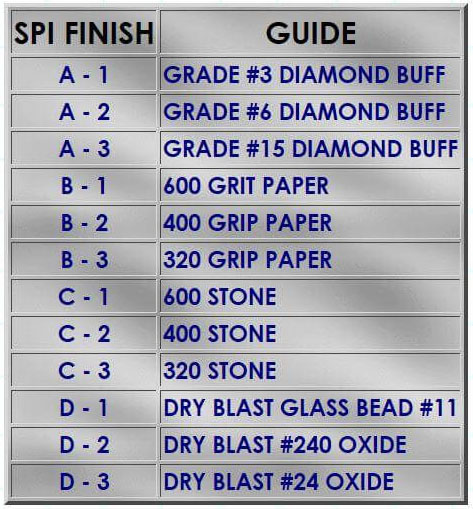
Relative to the surface texture of the part to be bonded, as molded, a textured surface will increase mechanical interlocking adhesion in addition to solvent cleaning and plasma pretreatment. Texture can be accomplished within the mold tool or manually using a Scotch-Brite pad. Even slightly textured surfaces such as C-3 (320 Stone) will be beneficial. SPI Finish guide is below.
For many polymer applications, 2-component, heat curable structural epoxy adhesives are ideal. Two-component epoxy consists of mixing an epoxy resin plus a hardener or procuring “duo-pak” applicators. Uniform, thin bond line thickness (0.002 – 0.007 inches) is preferred for optimal shear and tensile strength properties.
Full cure time is typically in the range of seven (7) days at 75°F, but is variable between applications and environment conditions. The speed of cure can be accelerated by adding heat (120-210°F) which results in additional polymerization and can give the epoxy better properties. Bake time and temperature is application specific. One-component epoxies eliminate the need for mixing but cure only with the addition of heat (normally 250-300 °F). By comparison, the working life of two-component epoxies is limited.
A common mispractice is to apply extra adhesive to improve bond strength. Excessive adhesive is detrimental and a leading cause of bonding failures. Epoxy adhesives have relatively high coefficients of thermal expansion. Bond lines need to be kept thin and uniform in thickness (typically 3-5 mil for maximum shear strength). The adhesive supplier can provide guidance for optimal bond line thickness for your application. Pre-measured “duo-pak” applicators will eliminate mixing problems and provide a more exact mix ratio. They also provide better stoichiometric chemical reactions, resulting in better strength & outgassing properties.
Polyamide, commonly known as Nylon, is a semi-crystalline polymer. The two most common grades are Nylon 6 and Nylon 6/6. Nylons are used in thousands of automotive, aerospace, medical and industrial applications where high temperature, solvent-proof, electrically-shielded parts are needed. While these characteristics are excellent for performance, poor surface wettability and moisture are bonding challenges for manufacturers.
Surface Properties
Nylon polymers are inherently difficult to bond because they are hydrophobic, chemically inert and possess poor surface wettability (i.e. low surface energy). Further, nylons are hygroscopic and will absorb moisture in excess of 3% of its mass of water from the atmosphere. Moisture, in and of itself, creates adhesion problems. The rate of moisture absorption is dependent upon time, relative humidity and temperature. Therefore, it is important to perform bonding processes as soon as possible following molding operations or package the parts tightly in non-poly bags with a desiccant. Proper drying procedure of nylon resins is critical to processing and part performance.
Wait a minute, on the face of it the above remarks would seem to be mutually contradictory. Is nylon hydrophobic or hydrophilic? The resolution of this apparent paradox comes in recognizing that the hydrophobic behavior of nylon is a surface property and the hydrophilic behavior is a bulk property.
Since nylon is an organic polymer it has a relatively low surface energy as do most polymers. This is a consequence of the surface chemistry and surface physics of polymers and other organics. However, the amide groups in the nylon chain attract water and they give rise to the hydrophilic behavior of this material in regard to BULK ABSORPTION of water. So, in the bulk nylon can behave as a hydrophilic material but on the surface it can exhibit hydrophobic behavior.
Cleaning and Pretreatments
Surface preparation is critical toward achieving high adhesive bond strength. Surfaces must be clean and contamination free from dirt, grease and oils. Low molecular weight materials (LMWM) such as silicones, mold release and anti-slip agents inhibit bonding. To suitably clean nylon surfaces and remove LMWM materials, use Toluene, Xylene, Acetone or MEK (in accordance with company solvent policy and state law). Alcohol is a weak solvent and only removes superficial dirt but not hydrocarbon contaminates. Proper technique must be used at all times including lint-free cloths and wearing powder-free hand protective gloves. Excess solvent creates weak boundary layers of un-removed chemicals leaving a haze build-up which will inhibit bonding.
Nylon bonding applications often require plasma pretreatment immediately following solvent cleaning. Gas-phase surface oxidation pretreatments for nylon include: electrical corona discharge, electrical atmospheric plasma, electrical air plasma, flame plasma and low pressure RF cold gas. These processes are characterized by their ability to generate “gas plasma”, an extremely reactive gas consisting of free electrons, positive ions, and other species. Chemical surface functionalization also occurs. In the science of physics, the mechanisms in which these plasmas are generated are different but their effects on surface wettability are similar. Each method is application specific and possesses advantages and/or limitations. For example, electrical pretreatments do not remove/clean all poly-aromatic hydrocarbons so it may be necessary to continue solvent cleaning. RF Cold Gas pretreatment will remove hydrocarbons, thus pre-cleaning is not necessary.
As a general rule, acceptable bonding adhesion is achieved when the surface energy of the substrate is approximately 10 dynes/cm greater than the surface tension of the liquid or adhesive. The surface energy of untreated nylon is approximately 40 dynes/cm. Therefore the desired post-treatment surface energy needs to be in the range of 50-54 dynes. In this situation, the liquid is said to “wet out” or adhere to the surface. A common method for measuring surface energy “wetting” is the use of calibrated dyne solutions in accordance with test method ASTM D2578.
Some paintable mold releases are available, but many other mold releases adversely affect paint adhesion. Various techniques may be required to remove these agents used to facilitate the separation of plastic parts from the molds. Wax-type mold releases can sometimes be removed by solvent cleaning, but this type of release is not recommended for parts to be painted. Solvent use is almost automatically is discouraged due to VOC emission restrictions and potential fire and health dangers with many solvents. Water-soluble mold releases are much preferred. Removal of these from the plastic surface is readily accomplished with ordinary aqueous detergent solutions.
Mold release agents may also be blended into plastic formulations; these are termed internal. Internal mold releases must be avoided whenever possible. Paintability may or may not be impaired immediately, and QA adhesion tests may show excellent initial adhesion. In some cases, however, internal mold releases have slowly migrated to the part surface and caused paint adhesion failure months after a part was painted. Plasticizers can be added to molding resins to increase impact strength, but often these can decrease paint adhesion just as mold releases do. Some plasticizers will slowly migrate to the surface and soften the interface between the plastic and the paint, resulting in adhesion loss. In this case, too, initial paint adhesion tests may be completely satisfactory, but subsequent lifting or separation of the paint film from the plastic surface occurs later. This can result in costly field failures and liability claims later, long after the part has been put into service.
Adhesion and abrasion durability of cured coatings and inks are two critical performance properties, and their measurement and control are essential for robust manufacturing. Adhesion and abrasion durability are discrete physical properties and commonly misdiagnosed during failure mode analysis. Poor adhesion and abrasion durability are leading causes of field failures, scrap and rework, lower profits, and dissatisfied customers. Techniques exist to measure these properties.
Adhesion Versus Abrasion
Optimal adhesion and abrasion durability (resistance) of cured coatings and inks onto plastic substrates must be achieved during manufacturing to produce high quality products that will endure throughout their service life. These properties can be interdependent but often are mutually exclusive. That is, cured coatings and inks can demonstrate good adhesion and poor abrasion, or poor adhesion and good abrasion. Proper testing and understanding of adhesion and abrasion ensures conformance to specifications and is invaluable in solving coating and printing problems. The identification of the failure mechanism, adhesion (cohesion), abrasion or both, will determine the engineering process solution. Historical test methods and new instrumentation exist to measure and analyze these properties.
Adhesion Test Methods
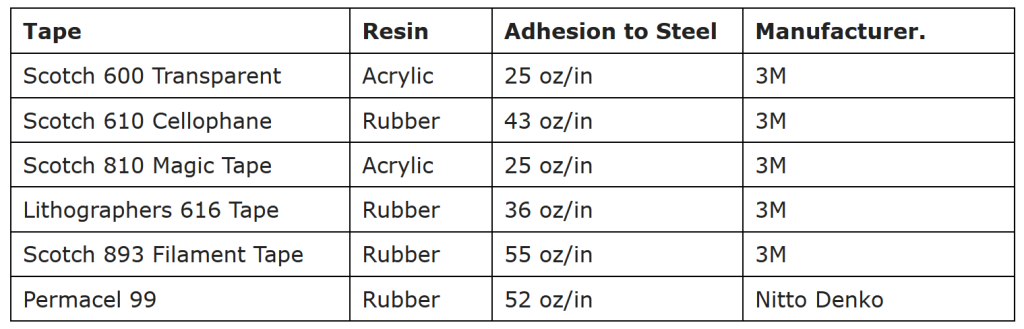
ASTM D 3359 Standard Test Method for Measuring Adhesion by Tape Test is historically perhaps the most well-known and widely used method for testing adhesion of coatings and inks because of its simplicity and low cost. The most basic procedure for conducting the tape test includes the following:
- Agreement on the selection of tape
- Coatings and inks must be completely cured before testing.
- Make X-cuts in the film per Method A or in a lattice pattern with either six or eleven cuts in each direction per Method B of the test.
- Remove and discard two laps of tape from the roll dispenser.
- Smooth the tape into place by finger, then rub firmly with an eraser on the end of a pencil or similar. The color under the tape is a good indicator of complete contact.
- Within 60-120 seconds of application, remove the tape by pulling the free end rapidly (do not jerk) back upon itself as close to an angle of 180 degrees as possible.
- Inspect the cut area for removal of coating from the substrate and rate the adhesion relative to predetermined descriptions and illustrations classifications (0B, 1B, 2B, 3B, 4B, 5B).