Abstract
Polymeric adhesion bonding problems are pervasive throughout the plastics industry. Two- and three-dimensional products often include joining plastic-to-plastic, plastic-to-metal and plastic-to-composite. Many plastics have a poor tendency to bond to other materials because of their inherent chemical structure and, therefore, require pretreatment.
Plasma surface pretreatments are used to promote adhesion between difficult-to-bond plastic substrates and adhesives, coatings, inks, and paints. This article discusses plasma science and the interaction mechanisms between a plasma and a polymer surface and further debunks common mythologies.
Introduction
“Gas-phase” plasma surface (modification) oxidation is the most common modification method and is proven to be highly effective, economical and environmentally safe. The selection of which method to utilize on any given application can be challenging, in part due to misconceptions and confusing terminology. Commonly known processes are electrical corona, remote-cold gas plasma, flame and UV/ozone (combinations). There are significant cost implications associated with bonding failure, including poor product field performance, scrap/rework, production inefficiencies and increased quality control inspection. Through understanding the basic science of contact angles, surface wetting and chemical activation, virtually any bonding problem can be successfully solved, even when using the most tough-to-bond polymeric and elastomeric materials.
Contact Angle, Surface Energy, and Wetting
The underlying reasons why many plastics are difficult to bond are because they are hydrophobic non-polar materials, chemically inert and possess poor surface wettability i.e., low surface energy. While these hydrophobic (repel water) performance properties are ideal for part designers who seek such properties, they are the nemesis for manufacturers needing to bond such materials. Robust adhesion bonding usually necessitates “hydrophilic” surfaces. For optimum adhesion to occur, an adhesive (coating, ink or paint) must thoroughly “wet out” the surface (adherend) to be bonded.
“Wetting out” means the liquid flows and covers a surface to maximize the contact area and the attractive forces between the adhesive and adherend bonding surface. For a liquid to effectively wet out a surface, the surface energy of the adhesive must be as low or lower than the surface energy of the adherend to be bonded. Alternatively, the surface energy of the substrate must be raised.
Consider a single liquid fluid droplet on a flat solid surface at rest (equilibrium). The angle formed by the solid surface and the tangent line to the upper surface at the end-point is called the contact angle; it is the angle (Θ) through the liquid between the tangent line at the contact point and the horizontal line of the solid surface. The bubble/droplet shape is due to the molecular forces by which all liquids, through contraction of the surface, tend to form the contained volume into a shape having the least surface area. The intermolecular forces that contract the surface are termed surface tension. Surface tension, a measurement of surface energy, is expressed in dynes/cm (SI N/m).
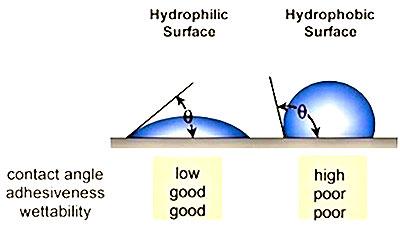
The higher the surface energy of the solid substrate relative to the surface tension of a liquid (water, printing inks, adhesives/encapsulation, coatings, etc.), the better will be its “wettability” and the smaller will be the contact angle (Figure 1). As a rule, acceptable bonding adhesion is achieved when the surface energy of a substrate is approximately 8 to 10 dynes/cm greater than the surface tension of the liquid.
For many applications it may only be necessary to examine the static equilibrium contact angle using dyne solutions in accordance to a documented test procedure. Application kits or “dyne pens/solutions” provide useful information, but they are not precise measurements of surface tension. Surface tension measurements can vary considerably by individual (technique) and the interpretation of the “center” liquid behavior.
Dyne pens/solutions are known to be directional indicators of significant differences in the surface tension and capable of identifying “good” and “bad” bondable surfaces at economical pricing. Bottled dyne solutions can be preferable instead of pens because of contamination issues due to multiple usage.
Dynamic Contact Angles (DCA)
Testing the fluid behavior of only the static contact angle can lead to misinterpretation of the liquid/solid interface results and the resolution of bonding problems. This is because industrial manufacturing production operations are more realistically dynamic conditions, not static. Thus, the dynamic contact angle (DCA) is important to understand (Figure 2).
Contact angles generally are affected by both changes in surface chemistry and changes in surface topography. The advancing contact angle is most sensitive to the low-energy (unmodified) components of the substrate surface, while the receding angle is more sensitive to the high-energy, oxidized groups introduced by surface pretreatments.

Thus, the receding angle actually is the measurement most characteristic of the modified component of the surface following pretreatments, as measured using dyne solutions. Therefore, it is important to measure both the advancing and receding contact angles on all surface-modified materials.
When a droplet is attached to a solid surface and the solid surface is tilted, the droplet will lunge forward and slide downward. The angles formed are respectively termed the advancing angle (Θa) and the receding angle (Θr). ASTM D724 describes methods for measuring DCAs using advanced equipment (optical tensiometers and goniometers) to analyze advancing and receding contact angles based on drop shape analysis and mass.
Chemical Surface Activation
There is a strong tendency for manufacturers to focus only on contact angles or other wettability measurements as the sole predictor for bonding problems and for conducting routine surface testing. Chemical surface functionality is equally important, whereby hydrophobic surfaces are activated into bondable hydrophilic surfaces. Gas-phase, “glow-discharge,” surface oxidation pretreatment processes are used for chemical surface activation. These processes are characterized by their ability to generate “gas plasma,” an extremely reactive gas consisting of free electrons, positive ions and other species. Plasmas often can be described as a fourth state of matter. As energy is supplied, solids melt into liquids, liquids vaporize into gases, and gases ionize into “plasmas.”
In the science of physics, the mechanisms in which these plasmas are generated are different, but their effects on surface wettability are similar. The basic chemical and physical reaction that occurs is free electrons, ions, metastables, radicals and UV generated in the plasma can impact a surface with energies sufficient to break the molecular bonds on the surface of most polymeric substrates. This creates very reactive free radicals on the polymer surface that, in turn, can form, cross-link, or in the presence of oxygen, react rapidly to form various chemical functional groups on the substrate surface. Polar functional groups that can form and enhance bondability include carbonyl (C=O), carboxyl (HOOC), hydroperoxide (HOO-), and hydroxyl (HO-) groups. Even small amounts of reactive functional groups incorporated into polymers can be highly beneficial to improving surface chemical functionality and wettability. Also, chemical primers/solvents and mechanical abrasion (including mold tool texture) can be utilized alone or in conjunction with pretreatments.

Commonly known processes are electrical corona discharge (also known as a dielectric barrier discharge), electrical atmospheric plasma, electrical air plasma, flame plasma, low-pressure RF cold gas and ultraviolet irradiation/ozone (combinations). Each method is application-specific and possesses unique advantages and potential limitations. Examination of the polymer (amorphous or semi-crystalline), plasma process, bonding agent and manufacturing process require careful examination and testing.
One important study conducted on polypropylene found that flame treatment appears to be the “shallowest” – that is, the oxygen incorporated by the treatment is most concentrated near the outer surface of the film. Corona and plasma treatments appear to penetrate somewhat deeper into the polymers. At the other extreme, the UV/ozone treatments reach farther into the bulk of the polymers.
Discharge conditions such as humidity effects are also important.1 It is known that certain pretreatments, and how they are applied, can deleteriously affect downstream manufacturing operations, e.g., delamination of toothpaste tubes during heat-sealing.
Factors Influencing Adhesion and Aging (Shelf Life)
The degree or quality of pretreatment for robust adhesion strength is affected by the cleanliness of the plastic surfaces. The surface must be clean to achieve optimal pretreatment and subsequent adhesion. Contamination sources on product surfaces that inhibit treatment include: dirt, dust, grease and oil. Low molecular weight materials such as silicones, mold release and anti-slip agents are particularly deleterious for bonding. Material purity is also an important factor. Further, certain soluble or nonsoluble compound agents used in pigment and dye colorants can adversely affect adhesion.
The shelf life (treatment aging degradation) of treated plastics depends on the type of resin, formulation and the ambient environment of the storage area. Shelf life of treated products is limited by the presence of low molecular weight oxidized materials (LMWOM) such as antioxidants, plasticizers, slip and antistatic agents, colorants and pigments, stabilizers, etc. Exposure of treated surfaces to elevated temperatures increases molecular chain mobility. The higher the chain mobility is, the faster the aging of the pretreatment. Plasma-treated surfaces age at different rates and to varying extents relative to factors with the surrounding environment. Aging characteristics and their storage shelf life are essential to manufacturing process operations. Activated surfaces may have a shelf life of hours, days, months or longer. It is recommended to bond, coat, paint or decorate products as soon as possible following pretreatment.
Plasma Surface Pretreatments
Classical electrical corona discharge (dielectric barrier discharge) is obtained using a generator and electrode(s) connected to a high-voltage source, a counter electrode at potential zero and a dielectric used as a barrier. That is, a high-frequency, high-voltage discharge (step-up transformer) creating a potential difference between two points requiring earth ground 35+kV and 20-25kHz. Custom electrode configurations allow for treating many different surface geometries – flat, contoured, recessed, isolated, etc.
One application example is a corona discharge treating system for electrical connectors in which a combination of pin and ball electrodes concomitantly treats 3D small diameter holes and flat exterior surfaces, US Patent US5051586 (1991)2. Ozone is produced in the plasma region as a result of the electrical discharge. Corona discharge has been found to be reasonably effective at cleaning the invisible hydrocarbon materials present after, e.g., solvent or detergent washing.
Myth: Atmospheric plasma is a low-cost replacement technology for corona discharge.
Fact: Corona discharge often is more effective for treating larger surface areas and at greater surface depth. In fact, many atmospheric plasma systems are more expensive.
Electrical “air plasma” is a corona discharge spot treatment (also termed blown air plasma/forced air corona/blown arc). This treatment head consists of two hook electrodes in close proximity to each other connected to a high-voltage transformer generating an electric arc of approximately 7 to 12kV, and lower frequency 50 to 60 cycles/sec (relative to electrical corona discharge). Then using forced air, a continuous electric arc produces a corona discharge, “plasma.” No positive ground is needed. This pretreatment process has virtually no cleaning capabilities. Ozone is produced.
Myth: Corona discharge spot treatment yields longer shelf life than flaming.
Fact: Scholarly studies show flame plasma on polyolefins produces longer post-treatment shelf life due to its relatively shallower depth of treatment.
Atmospheric plasma or electrical blown ion plasma (also termed focused corona plasma) utilizes a single narrow nozzle electrode, powered by an electrical generator and step-up transformer, and high-pressurized air in which intense focused plasma is generated within the treatment head and streams outward. This pretreatment process can clean dirt, debris and some hydrocarbons from the substrate, but not most silicones and slip agents. New research indicates fine etching of the surface can create new topographies for increased mechanical bonding. Ozone formation is negligible.
Myth: Atmospheric plasma is electrically potential-free.
Fact: Atmospheric plasma is better characterized as “low potential” unless definitively proven to be zero on any specific application. There are performance differences among equipment manufacturers.

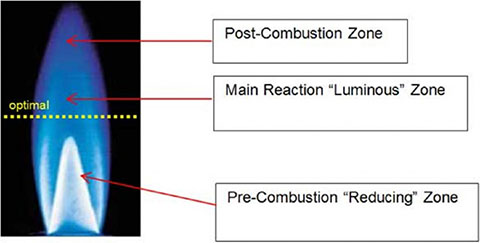
Flame plasma treatment uses the highly reactive species present in the combustion of air and hydrocarbon gas (to create the plasma). While flame treatment is exothermic, heat does not create the chemical functionality and improved surface wetting. Flaming will clean dirt, debris and some hydrocarbons from the substrate.
Flaming will not remove silicones, mold releases and slip agents. Flame treatment can impart higher wetting, oxidation and shelf life than electrical pretreatments due to its relatively shallower depth of treatment from the surface, 5 to 10nm. Ozone is not produced. When procuring flame treatment burners, compare ribbon vs. drilled port and the benefits of zero balanced regulators.
Myth: Flame treating is unsafe. False criticism arises from competitive equipment manufacturers.
Fact: For decades, flame treating has been used as a safe and effective technique across many industries.
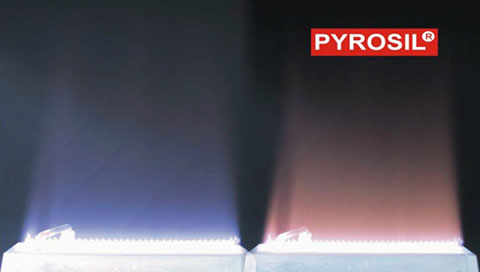

Pyrosil® is a newly evaluated pretreatment technique recently optimized for plastics (and metals) by Applied Surface Technologies LLC and The Sabreen Group Inc. The Pyrosil process is a proprietary flame technology developed by SURA Instruments GmbH 30 years ago and is highly effective for pretreating glass and ceramics.
Recently, Sabreen has independently advanced the process for plastic applications that require adhesive bonding, UV inkjet adhesion, decorative and functional coatings. The results on many products were excellent and, in some instances, outperformed traditional electrical discharge and flaming methods. Process control of the primer application and flame combustion is critical for optimal adhesion bonding.
The Pyrosil process is a combustion chemical vapor deposition technology leading to an amorphous silicate layer on the treated substrate. The surface is treated with the oxidizing part of a gas flame, which contains the precursor, an organosilicon compound. The precursor is pyrolysed (thermal decomposition) during the process and the formed ash is deposited as amorphous silicate on the surface, leading to an ultra-thin (20 to 40nm), strongly adhering coating.
In other words, a chemically highly reactive glass-like layer is formed. By evaporating a proprietary chemistry, which is mixed with propane and then burned – SiOx (silicon dioxide) is deposited onto substrates. SiOx creates high surface tension for improved hydrophilicity (wettability). Pyrosil is different than standard flame treatment. Easily scalable production systems can be custom built.
Myth: Pyrosil process is the same as traditional flame treatment.
Fact: The Pyrosil technology is different than flame treatment. It is a deposition process mixture of gas and silicone compounds.
Selecting a Plasma Surface Pretreatment
Recognize that each surface pretreament method is application-specific and may possess unique advantages and potential limitations. For robust results, consider the following factors:
- Polymers react differently to oxidation processes. The type of polymeric substrate and its end-use performance requirements are critical in determining the selection of pretreatment method.
- Is the substrate (product to be treated) conductive? For example, unassembled plastic electronic connector bodies – without metal contact pins – can be treated electrically, whereas assembled connectors may experience electrical arcing problems.
- Part geometry: Flat surfaces are more easily treated compared to deep recesses, extreme tapers and other shape irregularities. Wetting tests are difficult to conduct in small areas and on heavily textured surfaces.
- Material handling automation: Conductive belts and chains may cause electrical arcing with classical electrical corona discharge and spot treaters. Alternatively, consider using flame, cold gas plasma or low potential atmospheric plasma.
- Avoid overtreatment. Excessive plasma-oxidized surfaces may deleteriously affect downstream assembly processes such as heat sealing/welding.
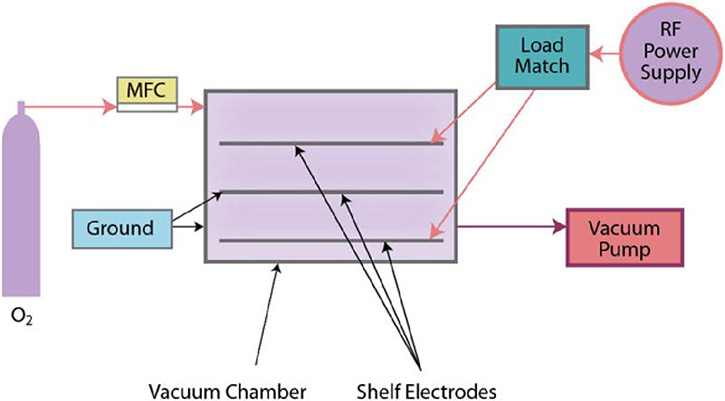
- All pretreatment equipment is not created equal. Examine the quality of constructed systems in action. For electrical treatment processes, observe the uniformity of the plasma discharge; for flame treatment, consider the differences between ribbon vs. drilled port burners and combustion system components; for cold gas plasma, examine the quality of the chamber construction, electrode shelves and particularly the manufacturer of the vacuum pump.
Polymeric bonding problems are widespread and not limited to adhesives. Significant cost implications are associated with adhesion bonding failure. Plasma surface pretreatments are the most common modification method, cost-effective and safe. The selection of which method to utilize on any given application can be challenging, in part due to misconceptions and confusing terminology.
Through understanding the science of contact angles, surface wetting, and chemical activation, virtually any bonding problem can be successfully solved, even when using the most challenging polymeric and elastomeric materials.
The Sabreen Group offers comprehensive onsite education and training on adhesion bonding processes, plasma pretreatment and advanced manufacturing techniques. Pretreatment equipment demonstration is conducted in-house, using actual production parts and real-time testing. Guaranteed Results.
Scott R. Sabreen is founder and president of The Sabreen Group, Inc., an engineering company specializing in secondary plastics manufacturing processes laser marking, surface pretreatments, bonding, decorating and finishing, and product security. Sabreen has been developing pioneering technologies and solving manufacturing problems for more than 30 years. He can be contacted at 972.820.6777 or by visiting www.sabreen.com or www.plasticslasermarking.com.
References
- Mark Strobel, Mary Jane Walzak, Josephine M. Hill, Amy Lin, Elizabeth Karbashewski & Christopher S. Lyons (2012) A comparison of gas-phase methods of modifying polymer surfaces, Journal of Adhesion Science and Technology, 9:3, 365-383, DOI: 10.1163/156856195X00554
- Scott R. Sabreen, The Sabreen Group, Inc., “Surface Wetting Pretreatment Methods,” Plastics Decorating magazine, 2002



